Generic Drugs: What They Are, How They Work, and What You Need to Know
When you hear generic drugs, pharmaceutical products that contain the same active ingredients as brand-name drugs but are sold under their chemical name. Also known as generic medications, they are legally required to work the same way, in the same amount, and at the same speed as their branded counterparts. The big difference? Price. A generic version of a popular pill can cost 80% less—sometimes even more. And yet, millions of people switch to them every year without noticing a difference in how they feel.
But here’s what most people don’t realize: brand name drugs, medications originally developed and marketed by pharmaceutical companies under a patent. Also known as originator drugs, they carry the cost of research, marketing, and patent protection isn’t just about the pill inside. It’s about the name on the bottle. Once a patent expires, other manufacturers can legally make the same drug. That’s when drug substitution, the practice of replacing a brand-name drug with a generic version at the pharmacy. Also known as generic switching, it’s standard in most U.S. pharmacies unless the doctor or patient says no kicks in. Pharmacists can swap them automatically in most states—unless you specifically ask for the brand. That’s why you might get a different-looking pill from one refill to the next, even if the name on the label hasn’t changed.
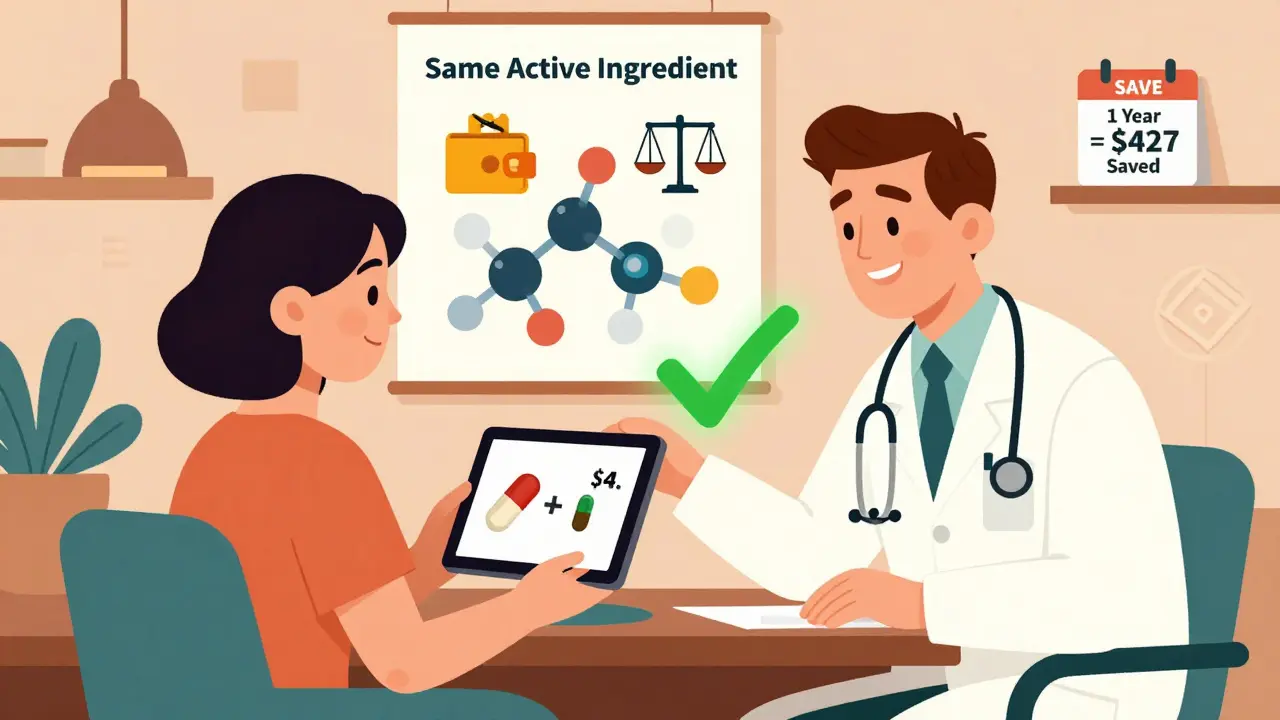
Some folks worry that generics aren’t as strong or don’t work as well. That’s not true. The FDA requires them to meet the same strict standards for purity, strength, and performance. The only differences allowed are in inactive ingredients—like fillers or dyes—which rarely affect how the drug works. But here’s the catch: medication cost, the total expense a patient pays for a drug, including out-of-pocket and insurance contributions isn’t always about the pill itself. If you’re on multiple prescriptions, even small savings add up fast. A $50 brand-name drug becomes $10 as a generic. Multiply that by five pills a week, and you’re saving over $1,000 a year. That’s why doctors increasingly start patients on generics unless there’s a clear medical reason not to.
Still, not every situation is straightforward. Some patients report feeling different after switching—maybe a little more tired, or less clear-headed. That’s not always the drug’s fault. Sometimes it’s the change in pill size, shape, or even the brand of inactive ingredients. Rarely, it’s a real issue with bioequivalence. That’s why it’s important to talk to your doctor or pharmacist if something feels off after a switch. And yes, there are cases where the brand is still preferred—like with narrow-therapeutic-index drugs such as warfarin or thyroid meds—where even tiny variations matter.
What you’ll find below is a collection of real-world stories and comparisons about drugs you might already be taking. From how generic drugs stack up against Zovirax, Levitra, or Provera, to the legal risks doctors face when prescribing them, to how much you’re really saving on HIV meds or hormone therapies. These aren’t theory pieces. They’re practical, no-fluff guides written by people who’ve been there. Whether you’re trying to cut costs, understand why your pill looks different, or just want to know if you’re getting the same effect—you’ll find answers here.
Medication Costs: How Coupons, Generics, and Prior Authorizations Affect Your Out-of-Pocket Expenses
15 Comments
Learn how coupons, generics, and prior authorizations impact your prescription costs in 2026. Discover real ways to save money on meds with practical steps and new Medicare changes.
Read MoreAuthorized Generics: Same Drug, Different Label
8 Comments
Authorized generics are identical to brand-name drugs but sold under a different label. Learn how they work, why they’re cheaper, and how to spot them - without sacrificing quality or safety.
Read MoreHow to Ask Your Doctor About Generic Alternatives for Lower-Cost Medications
13 Comments
Learn how to ask your doctor about generic alternatives to save money on prescriptions without sacrificing effectiveness. Most generics are just as safe and effective as brand-name drugs, and switching can cut your costs by up to 95%.
Read MoreModified-Release Formulations: What You Need to Know About Bioequivalence Standards
13 Comments
Modified-release formulations require complex bioequivalence testing to ensure safety and effectiveness. Learn how regulators assess extended-release generics, why alcohol testing matters, and what happens when a pill doesn't release as designed.
Read More